Lithium Brines “Battery Soup” Part One
Lithium Brines “Battery Soup” Part One
I’m not going to describe the uses of lithium in any great detail. Suffice it to say, lithium is increasingly used in the “green” battery industry. Lithium producers, near-producers and explorers provide broad commentary on lithium on their websites and/or in their company presentations.
Think lithium, think new age batteries, EV’s, green energy, responsible/sustainable development.
In fact, lithium has a wide range of applications, batteries, laptops, mobile phones, digital cameras, medicines, ceramics, glasses (cook-top glass for example), metallurgy, fusion energy, cement and in special lubricants.
Its’ the “rise and rise” of lithium.
The aim of this article is to describe lithium brines, what they are, how they develop and where they occur. The aim is to better understand lithium brine related exploration results that are released to the market by explorers and near-producers.
Lithium
Lithium is a soft and silvery alkali metal. As an alkali metal, it is very reactive and flammable. Lithium has an atomic number of 3 (hydrogen = 1, helium = 2 which are both gases). In normal conditions lithium is the least dense element/metal known.
Lithium is a relatively rare element and does not occur in pure form naturally. It almost always occurring as ionic compounds (salts) commonly in/as minerals. Lithium occurs in the oceans at levels between 0.14ppm and 0.25ppm. This compares to chlorine levels of ±20,000ppm and sodium levels of ±10,000ppm. Lithium occurs in the earth’s crust at averaged levels of between 20ppm and 70ppm.
Ppm = part per million.
Lithium is mined from (hard rock) pegmatites, (soft rock) clays and from (water) brines. Hard rock lithium minerals include spodumene and petalite. A soft rock lithium mineral is hectorite.
In Part One of this series, I am going to talk about lithium that occurs in brines – lithium brines.
What is a “brine”?
A brine is water that has high concentrations of salts, among the most common salts being sodium chlorite (NaCl) [table salt] and calcium chlorite (CaCl2).
In nature, brines can develop under the right conditions from seawater. Such conditions may prevail in lagoons, estuaries, etc… where the water flow is restricted/becalmed and shallow with high levels of evaporation.
Brines can also develop under the right conditions from groundwater. Such conditions may prevail in closed shallow lakes, again, with high levels of evaporation.
As a point of interest, sediments (or rock) that contain high levels of minerals formed by the process of evaporation are called evaporites. Evaporites derived from seawater brines tend to contain high levels of halite (NaCl) and gypsum (CaSO4.2H2o). Evaporites derived from groundwater tend contain a more variable assemblage of minerals reflecting local geology and environment, halite, anhydrite, carbonates, gypsum, fluoride-salts, organic halides, sulphate-salts, and borax (general formula: Na2H20B4O17). More on Borax in a later article in this series.
What is a “lithium brine”?
A lithium brine is simply a brine with high levels of lithium. There’s nothing much more to it. Lithium levels in lithium brines can range from a low of 200milligrams/litre to around 1,500milligrams/litre.
1milligram/litre = 1ppm.
200milligrams/litre = 200ppm. 1,500milligrams/litre = 1,500ppm.
What are the conditions for naturally occurring lithium brines to develop?
Looking backwards, so to speak, from where known lithium brines are located, the conditions for the development of a lithium brine can be unpicked.
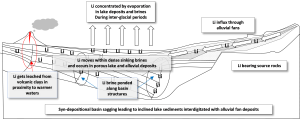
The vast majority of lithium brines are located in arid regions that have dry (salt) lakes within a closed drainage system in a geothermally active volcanic geological setting. The one location that keeps appearing in lithium articles and presentations is the Lithium Triangle spanning three countries Bolivia, Argentina and Chile. I’ll talk about that in Part Two of this series.
There are several names for a closed drainage system including, internal drainage system, and endorheic drainage system. It is a drainage system that does not flow to the ocean.
A closed (endorheic) basin is a low point in a closed drainage system from which no water flows out (into rivers or oceans).
A close (endorheic) lake, also called a terminal lake, is pretty much the same as a closed basin, but perhaps on a smaller scale. No water flows out of it, only into it.
#1 Criteria: The occurrence of a closed lake (endorheic lake = terminal lake = [less formally] salt lake). A lithium explorer will highlight the point that their lithium project has a closed lake(s) or endorheic lake(s).
Next criteria…
The need for a geothermal gradient and/or active volcanics also appears necessary based on the location of known lithium brines. The presence of a hot spring or hot spring system is an indication of a shallow geothermal gradient and active volcanism.
The percolation of hot groundwater through volcanic rocks with high average lithium levels, provides the circumstance in which lithium is liberated from the bedrock and drawn into the heated ground water. Convection, leakages along faults, and evaporation all play a part in lithium becoming enriched in closed lake “trapped” groundwater and sediments.
#2 Criteria: Volcanic rocks as the source of the lithium and hot water to liberate the lithium from these rocks. Lithium exploration companies will emphasise the volcanic setting of the project area and the occurrence of hot springs.
Next criteria…
So, an exploration company has a closed lake and hot spring setting. The third criteria is the natural development of a brine within the closed lake. As mentioned above for a brine to develop evaporation is required. High rates of evaporation occur in areas with intense solar radiation, no to very little precipitation (rain), and a regime of strong winds.
#3 Criteria: Lithium brines develop in areas with high evaporation rates. Lithium exploration companies will emphasise the arid conditions of their project area.
And so …the lithium pathway from rock to brine is a shallow geothermal heat source, deep chemical leeching of volcanic rocks (and sediments derived from volcanic rocks), trapped groundwater, ponded in closed lakes, and evaporation.
There is another important reason why evaporation is required.
I don’t normally stray into ore processing, as I have mentioned previously, exploration is my field of experience, not mining.
Evaporation is not only needed for the “natural” development of lithium brines, but an integral part of the commercial development of lithium brines.
Onsite concentration of lithium in brines is achieved through various ponding and evaporation processes. These processes can take over 12 months to complete. Lithium levels in such concentrates can rise to 1% to 6%.
1% = 10,000 ppm = 10,000 milligrams/litre.
6% = 60,000 ppm = 60,000 milligrams/litre.
A simplified explanation of lithium extraction onsite:
Lithium brine is pumped to the surface to evaporate in a pond. A salt crust is developed (and usually discarded). This is the ‘first pan”. The brine (less the minerals that in the first crust) is pumped to another pond, the “second pan.” A second crust is developed, and the residual brine is now enriched in potassium sulphate, sodium, magnesium and lithium salts (boron salts too). This process is driven by nature – by solar radiation (sunshine) and evaporation.
The lithium-rich brine is pumped to a lithium carbonate processing plant (onsite) where it is mixed with sodium carbonate. In a simple exchange of Li ó Na. Lithium carbonate is produced.
Li producers often sell lithium carbonate after it is filtered, washed, dried and packaged.
Alternatively, lithium carbonate can be further treated to produce lithium hydroxide. It is not a necessity for this process to be done onsite.
#4 [Commercial] Criteria: Evaporation to produce a lithium salt that is the feed material into a lithium carbonate processing plant onsite to produce lithium carbonate. Explorers looking to become producers will emphasise that their project area has broad expanses of reservoirs/ponds exposed to the hot arid sun, sufficient room, and the natural environment to produce lithium carbonate.
A Lithium Brine Exploration Model
Given the above-described criteria for the natural enrichment of lithium in brines (closed lake, lithium-bearing bedrock, heat source, evaporation), I present an Exploration Model created in collaboration with Jayson Meyers of Resource Potentials.
In the next article on Lithium Brine I’ll talk about the Lithium Triangle.